Keywords
Abstract
Purpose: To report the clinical experiences of author AH, who calculated that modest stepwise lowering of arterial blood pressure can reverse (i) re-emergent diabetic retinopathy (DR) caused by antiplatelet and anticoagulant agents, even in the presence of continued use of the latter necessary agents, or (ii) DR induced by common or severe hypertension and so, (iii) simultaneously treat both of AH’s vascular and ocular medical conditions.
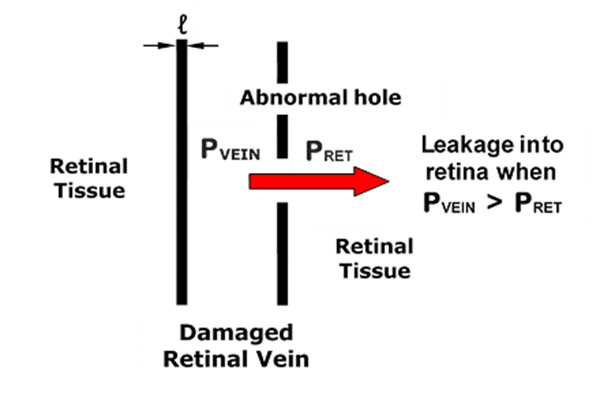
Methods: In instances of DR and visual impairment with evidence of exudate formation, blood pressure adjustments were applied, based on mathematical models of retinal exudate production developed by one of the authors (AH). Specifically, the model was used to calculate a critical arterial blood pressure below which retinal exudate formation should cease. Antihypertensive agents were then increased gradually until the desired lower target blood pressure was achieved and DR eliminated. Optical coherence tomography (OCT) was used to test for therapeutic effectiveness.
Results: In four different clinical situations, which included blood thinners or hypertension, control of retinal exudate formation and elimination of re-emergent DR was achieved solely by blood pressure lowering and confirmed (with OCT) by return, to normal, of retinal measurements and vision.
Conclusion: While the evidence presented here is derived from clinical examples in one person and not from a statistically justified large study, this approach to the control of retinal exudate formation offers very effective unintrusive management of a common vision-threatening aspect of DR. In particular, this approach avoids laser treatments and the challenging experience of commonly administered intraocular injections. Clinical and mathematical evidence is presented that treatment with abundant vitamin B1 (300 mg) and vitamin D results in partial cure of DR. A cure to DR has not been reported before.
Future perspectives: The reversal of DR and potentially age-related macular degeneration (ARMD), with safe and simple measures, is an incredibly worthy management goal for these two very common conditions. The possibility demands urgent evaluation with what should be zero- or low-risk clinical trials.
References
Lowe GD, Ghafour IM, Belch JJ, Forbes CD, Foulds WS, MacCuish AC. Increased blood viscosity in diabetic proliferative retinopathy. Diabetes Res. 1986;3(2):67-70. https://www.ncbi.nlm.nih.gov/pubmed/3698481
Turczynski B, Michalska-Malecka K, Slowinska L, Szczesny S, Romaniuk W. Correlations between the severity of retinopathy in diabetic patients and whole blood and plasma viscosity. Clin Hemorheol Microcirc. 2003;29(2):129-137. https://www.ncbi.nlm.nih.gov/pubmed/14610308
Irace C, Carallo C, Scavelli F, De Franceschi MS, Esposito T, Gnasso A. Blood viscosity in subjects with normoglycemia and prediabetes. Diabetes Care. 2014;37(2):488-492. https://doi.org/10.2337/dc13-1374
Irace C, Scarinci F, Scorcia V, et al. Association among low whole blood viscosity, haematocrit, haemoglobin and diabetic retinopathy in subjects with type 2 diabetes. Br J Ophthalmol. 2011;95(1):94-98. https://doi.org/10.1136/bjo.2009.172601
Helfgott A, Helfgott ER, Mullany S. Using mathematics to avoid blindness in diabetics. Journal for Modeling in Ophthalmology. 2018;1:42-70.
Thornalley PJ, Babaei-Jadidi R, Al Ali H, et al. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50(10):2164-2170. https://doi.org/10.1007/s00125-007-0771-4
Caro CG, Pedley TJ, Shroter RC, Seed WA. The mechanics of the circulation. 2nd Edition ed.: Cambridge University. Press; 2012. 523 p.
Klabunde RE. Cardiovascular Physiological Concepts. Third ed. 2021.
Lowe GD, Lowe JM, Drummond MM, et al. Blood viscosity in young male diabetics with and without retinopathy. Diabetologia. 1980;18(5):359-363. https://doi.org/10.1007/BF00276814
Melli M, Poggi M, Codeluppi L, Baraldi P, Torlai F, Peduzzi M. [Blood viscosity and erythrocyte deformability in diabetic retinopathy]. Ric Clin Lab. 1983;13 Suppl 3:371-374. https://www.ncbi.nlm.nih.gov/pubmed/6673016
Park JH, Kim JY, Baik JS, Park JY, Nam HS, Han SW. Prior antithrombotic use is significantly associated with decreased blood viscosity within 24 hours of symptom onset in patients with acute ischemic stroke. J Neurocrit Care. 2019;12:85-91.
Song SH, Kim JH, Lee JH, Yun YM, Choi DH, Kim HY. Elevated blood viscosity is associated with cerebral small vessel disease in patients with acute ischemic stroke. BMC Neurol. 2017;17(1):20. https://doi.org/10.1186/s12883-017-0808-3
Cowan AQ, Cho DJ, Rosenson RS. Importance of blood rheology in the pathophysiology of atherothrombosis. Cardiovasc Drugs Ther. 2012;26(4):339-348. https://doi.org/10.1007/s10557-012-6402-4
Furukawa K, Abumiya T, Sakai K, et al. Increased Blood Viscosity in Ischemic Stroke Patients with Small Artery Occlusion Measured by an Electromagnetic Spinning Sphere Viscometer. J Stroke Cerebrovasc Dis. 2016;25(11):2762-2769. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.07.031
Vekasi J, Koltai K, Gaal V, Toth A, Juricskay I, Kesmarky G. The effect of aspirin on hemorheological parameters of patients with diabetic retinopathy. Clin Hemorheol Microcirc. 2008;39(1-4):385-389. https://www.ncbi.nlm.nih.gov/pubmed/18503149
Ciuffetti G, Lombardini R, Pirro M, Lupattelli G, Mannarino E. Clopidogrel: hemorheological effects in subjects with subclinical atherosclerosis. Clin Hemorheol Microcirc. 2001;25(1):31-39. https://www.ncbi.nlm.nih.gov/pubmed/11790868
Zhang X, Chen J, Ding M, Shang N. Aspirin plus clopidogrel for angina pectoris in coronary heart disease patients. International Journal of Clinical and Experimental Medicine. 2018;11(12):3528-13534.
De Scalzi M, De Leonardis V, Citi S, Cinelli P. Relationship between systolic time intervals and arterial blood pressure. Clin Cardiol. 1986;9(11):545-549. https://doi.org/10.1002/clc.4960091104
Kreyszig E. Advanced engineering mathematica. 9th ed.: John Wiley &Sons, Inc; 2006.
Pacal L, Kuricova K, Kankova K. Evidence for altered thiamine metabolism in diabetes: Is there a potential to oppose gluco- and lipotoxicity by rational supplementation? World J Diabetes. 2014;5(3):288-295. https://doi.org/10.4239/wjd.v5.i3.288
Beltramo E, Mazzeo A, Porta M. Thiamine and diabetes: back to the future? Acta Diabetol. 2021;58(11):1433-1439. https://doi.org/10.1007/s00592-021-01752-4
Chen Q, Okada S, Okeda R. Causality of parenchymal and vascular changes in rats with experimental thiamine deficiency encephalopathy. Pathol Int. 1997;47(11):748-756. https://doi.org/10.1111/j.1440-1827.1997.tb04452.x
Gui QP, Zhao WQ, Wang LN. Wernicke's encephalopathy in nonalcoholic patients: clinical and pathologic features of three cases and literature reviewed. Neuropathology. 2006;26(3):231-235. https://doi.org/10.1111/j.1440-1789.2006.00665.x
Luong KV, Nguyen LT. The impact of thiamine treatment in the diabetes mellitus. J Clin Med Res. 2012;4(3):153-160. https://doi.org/10.4021/jocmr890w
Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-690. https://doi.org/10.1111/j.1742-1241.2011.02680.x
Payne JF, Ray R, Watson DG, et al. Vitamin D insufficiency in diabetic retinopathy. Endocr Pract. 2012;18(2):185-193. https://doi.org/10.4158/EP11147.OR
Castillo-Oti JM, Galvan-Manso AI, Callejas-Herrero MR, Vara-Gonzalez LA, Salas-Herrera F, Munoz-Cacho P. Vitamin D Deficiency Is Significantly Associated with Retinopathy in Type 2 Diabetes Mellitus: A Case-Control Study. Nutrients. 2021;14(1). https://doi.org/10.3390/nu14010084
Alcubierre N, Valls J, Rubinat E, et al. Vitamin D Deficiency Is Associated with the Presence and Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. J Diabetes Res. 2015;2015:374178. https://doi.org/10.1155/2015/374178
Reddy GB, Sivaprasad M, Shalini T, et al. Plasma vitamin D status in patients with type 2 diabetes with and without retinopathy. Nutrition. 2015;31(7-8):959-963. https://doi.org/10.1016/j.nut.2015.01.012
Yoshihara T, Zaitsu M, Shiraishi F, et al. Influence of genetic polymorphisms and habitual caffeine intake on the changes in blood pressure, pulse rate, and calculation speed after caffeine intake: A prospective, double blind, randomized trial in healthy volunteers. J Pharmacol Sci. 2019;139(3):209-214. https://doi.org/10.1016/j.jphs.2019.01.006
Kabatas N, Dogan AS, Yilmaz M, et al. Association between age-related macular degeneration and 25(OH) vitamin D levels in the Turkish population. Arq Bras Oftalmol. 2022;85(1):7-12. https://doi.org/10.5935/0004-2749.20220002
Merle BM, Silver RE, Rosner B, Seddon JM. Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: a prospective cohort study. Am J Clin Nutr. 2016;103(4):1135-1144. https://doi.org/10.3945/ajcn.115.117606
Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. https://doi.org/10.1016/j.ophtha.2012.09.006