Keywords
Abstract
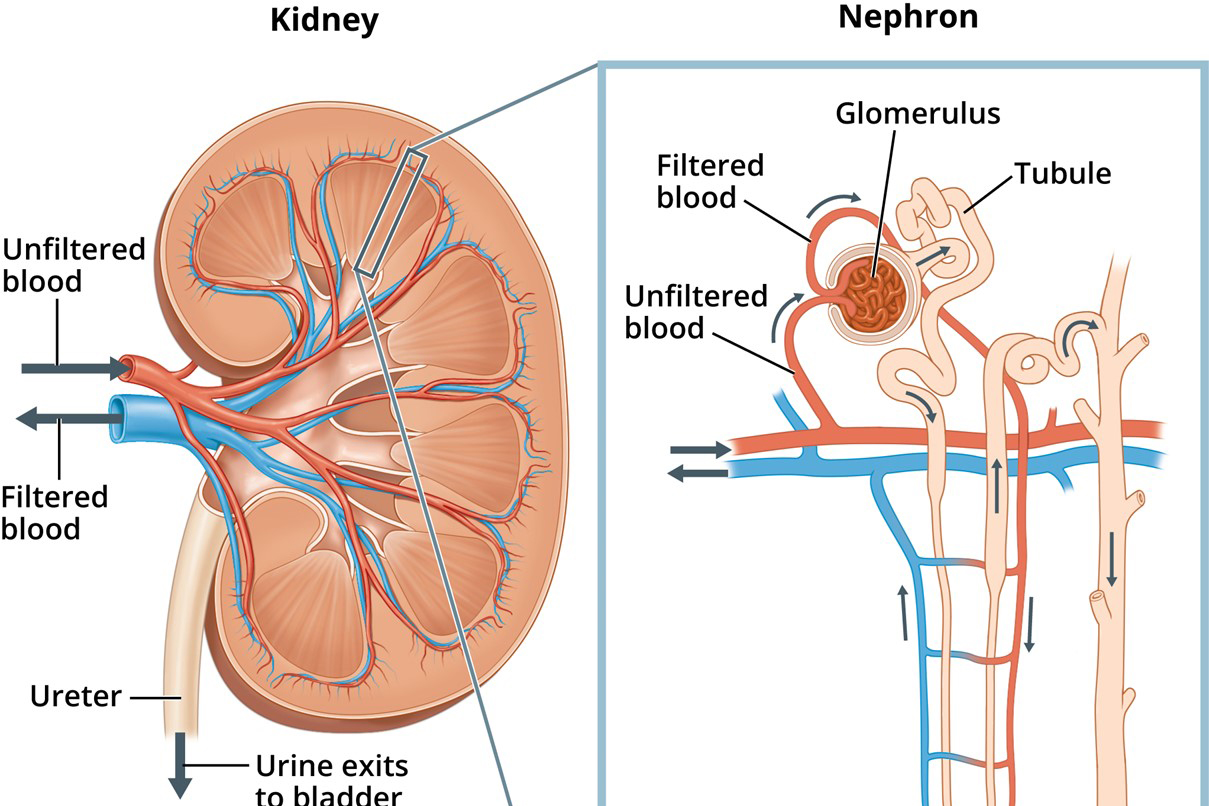
Purpose: The purpose of this review is to describe computational models that have been developed for studying kidney function and howthese models may be adapted to study the eyes.
Methods: We derive equations for modeling solute andwater transport across epithelial cell membranes in the kidney. These equations describe mass conservation, as well membrane transport via cotransporters, exchangers, and primary active transport.
Results: Wedescribe howcomputational models of renal transport have been applied to investigate kidney function in physiological and pathophysiological conditions.
Conclusion: The computational models herein described for the kidney may be adapted to study ocular functions and dysfunction.
References
Eaton DC, Pooler J, Vander AJ. Vander’s renal physiology. en. 7th. OCLC: 340936292. New York: McGraw-Hill Medical, 2009; ISBN: 978-0-07-161304-0. Available from: http://www.accessmedicine.com/resourceTOC.aspx?resourceID=57, visited on 11/13/2021.
Layton AT, Edwards A. Mathematical Modeling in Renal Physiology. eng. 1st ed. 2014. Lecture Notes on Mathematical Modelling in the Life Sciences. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014. ISBN: 978-3-642-27367-4. https://doi.org/10.1007/978-3-642-27367-4.
Weinstein AM. A kinetically defined Na+/H+ antiporter within a mathematical model of the rat proximal tubule. Journal of General Physiology, May 1995;105(5): 617–641. https://doi.org/10.1085/jgp.105.5.617.
Layton AT, Vallon V, Edwards A. A computational model for simulating solute transport and oxygen consumption along the nephrons. eng. American journal of physiology. Renal physiology, 2016;311(6): https://doi.org/10.1152/ajprenal.00293.2016.
Okada Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell biochemistry and biophysics, 2004;41(2): 233–258.
Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. International review of cytology, 1995;161, 173–262.
Edwards A, Layton AT. Cell volume regulation in the proximal tubule of rat kidney. Bulletin of mathematical biology, 2017;79(11): 2512–2533.
Völkl H, Lang F. Effect of potassium on cell volume regulation in renal straight proximal tubules. The Journal of membrane biology, 1990;117(2): 113–122.
Barriere H, Rubera I, Belfodil R, Tauc M, Tonnerieux N, Poujeol C, et al. Swelling-activated chloride and potassium conductance in primary cultures of mouse proximal tubules. Implication of KCNE1 protein. The Journal of membrane biology, 2003;193(3): 153–170.
Mitchell CH, Fleischhauer JC, Stamer WD, Peterson-Yantorno K, Civan MM. Human trabecular meshwork cell volume regulation. American Journal of Physiology-Cell Physiology, 2002;283(1): C315–C326.
Soto D, Comes N, Ferrer E, Morales M, Escalada A, Palés J, et al. Modulation of aqueous humor outflow by ionic mechanisms involved in trabecular meshwork cell volume regulation. Investigative ophthalmology & visual science, 2004;45(10): 3650–3661.
Sandberg K, Ji H. Sex differences in primary hypertension. Biology of sex differences, 2012;3(1): 1–21.
Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, et al. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. American journal of hypertension, 1995;8(10): 978–986.
Ouchi Y, Share L, CroftonJT, Iitake K, Brooks DP. Sex difference in the development of deoxycorticosteronesalt hypertension in the rat. Hypertension, 1987;9(2): 172–177.
Chen Y, Sullivan JC, Edwards A, Layton AT. Sex-specific computational models of the spontaneously hypertensive rat kidneys: factors affecting nitric oxide bioavailability. American Journal of Physiology-Renal Physiology, 2017;313(2): F174–F183.
Hilliard LM, Sampson AK, Brown RD, Denton KM. The “his and hers” of the renin-angiotensin system. Current hypertension reports, 2013;15(1): 71–79.
Leete J, Gurley S, Layton AT. Modeling sex differences in the renin angiotensin systemand the efficacy of antihypertensive therapies. Computers & chemical engineering, 2018;112, 253–264.
Pollow DP, Uhrlaub J, Romero-Aleshire MJ, et al. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension, 2014;64(2): 384–390.
Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension, 2014;64(3): 557–564.
Munger K, Baylis C. Sex differences in renal hemodynamics in rats. American Journal of Physiology-Renal Physiology, 1988;254(2): F223–F231.
Sabolić I, Asif AR, Budach WE,Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflügers Archiv-European Journal of Physiology, 2007;455(3): 397–429.
Veiras LC, Girardi AC, Curry J, Pei L, Ralph DL, Tran A, et al. Sexual dimorphic pattern of renal transporters and electrolytehomeostasis. Journal of the American Society of Nephrology, 2017;28(12): 3504–3517.
Li Q, McDonough AA, Layton HE, Layton AT. Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling andanalysis. American Journal of Physiology-Renal Physiology, 2018;315(3): F692–F700.
Sabolić I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. American Journal of Physiology-Cell Physiology, 2012;302(8): C1174–C1188.
Hu R, McDonough AA, Layton AT. Functional implications of the sex differences in transporter abundance along the rat nephron: modeling and analysis. eng. American journal of physiology. Renal physiology. Sex and Gender in Renal Health and Function, 2019;317(6):F1462–F1474. https://doi.org/10.1152/ajprenal.00352.2019
Hu R, McDonough AA, Layton AT. Sex differences in solute transport along the nephrons: effects of Na+ transport inhibition. eng. American journal of physiology. Renal physiology, 2020;319(3): F487–F505. https://doi.org/10.1152/ajprenal.00240.2020.
Hu R, McDonough AA, Layton AT. Sex differences in solute and water handling in the human kidney: Modeling and functional implications. en. iScience, June 2021;24(6): 102667. https://doi.org/10.1016/j.isci.2021.102667.
Swapnasrita S, Carlier A, Layton AT. Sex-Specific Computational Models of Kidney Function in Patients With Diabetes. Frontiers in Physiology, 2022;13. ISSN: 1664-042X. Available from: https://www. frontiersin.org/article/10.3389/fphys.2022.741121, visited on 03/04/2022.
West CA, Sasser JM, Baylis C. The enigma of continual plasma volume expansion in pregnancy: critical role of the renin-angiotensin-aldosterone system. American Journal of Physiology-Renal Physiology, Oct. 2016;311(6): F1125–F1134. https://doi.org/10.1152/ajprenal.00129.2016.
Souza AMA de, West CA. Adaptive remodeling of renal Na+ and K+ transport during pregnancy. en-US. Current Opinion in Nephrology and Hypertension, Sept. 2018;27(5): 379–383. ISSN: 1062-4821. https://doi.org/10.1097/MNH.0000000000000441.
Atherton J, Pirie SC. The effect of pregnancy on glomerular filtration rate and salt and water reabsorption in the rat. Journal of Physiology, Jan. 1981;319, 153–164.
Stadt MM, Layton AT. Adaptive changes in single-nephron GFR, tubular morphology, and transport in a pregnant rat nephron: modeling and analysis. American Journal of Physiology-Renal Physiology, Feb. 2022;322(2): F121–F137. https://doi.org/10.1152/ajprenal.00264.2021.
Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. en. Frontiers in Physiology, Aug. 2018;9, 1091. https://doi.org/10.3389/fphys.2018.01091
Abo S, Smith D, Stadt M, Layton A. Modelling Female Physiology fromHead to Toe: Impact of Sex Hormones, Menstrual Cycle, and Pregnancy. en. Journal of Theoretical Biology, Feb. 2022; 111074. https://doi.org/10.1016/j.jtbi.2022.111074.
Khong EWC, Chan HHL, Watson SL, Lim LL. Pregnancy and the eye. en-US. Current Opinion in Ophthalmology, Nov. 2021;32(6): 527–535. https://doi.org/10.1097/ICU.0000000000000778
Chawla S, Chaudhary T, Aggarwal S, Maiti GD, Jaiswal K, Yadav J. Ophthalmic considerations in pregnancy. en. Medical Journal Armed Forces India, July 2013;69(3): 278–284. https://doi.org/10.1016/j.mjafi.2013.03.006
Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. American Journal of Physiology-Renal Physiology, 2015;308(12): F1343–F1357.
O’Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. American journal of physiology-renal physiology, 2015;309(3): F227–F234.
Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vision research, 2017;139, 72–81.
Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. II. Functional implications of three-dimensional architecture. American Journal of Physiology-Renal Physiology, 2011;300(2): F372–F384.
Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. I. Formulation and base-case results. American Journal of Physiology-Renal Physiology, 2011;300(2):F356–F371.
Layton AT, Layton HE. A region-based model framework for the rat urine concentrating mechanism. Bulletin of mathematical biology, 2003;65(5): 859–901.
Chen J, Sgouralis I, Moore LC, Layton HE, Layton AT. Amathematical model of the myogenic response to systolic pressure in the afferent arteriole. American Journal of Physiology-Renal Physiology, 2011;300(3): F669–F681.
Layton AT. Feedback-mediated dynamics in a model of a compliant thick ascending limb. Mathematical biosciences, 2010;228(2): 185–194.
Layton AT, Moore LC, Layton HE. Multistable dynamics mediated by tubuloglomerular feedback in a model of coupled nephrons. Bulletin of mathematical biology, 2009;71(3): 515–555.
Layton AT, Edwards A, Vallon V. Adaptive changes in GFR, tubular morphology, and transport in subtotal nephrectomized kidneys: modeling and analysis. American Journal of Physiology-Renal Physiology, 2017;313(2): F199-F209.
Hu R, Layton A. A computational model of kidney function in a patient with diabetes. International journal of molecular sciences, 2021;22(11): 5819.
Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. American Journal of Physiology-Renal Physiology, 2018;314(5): F969–F984.
Avtar R, Srivastava R, Nigam D. A mathematical model for solute coupled water transport in the production of aqueous humor. Applied mathematical modelling, 2008;32(7): 1350–1369.
Cheng X, Pinsky PM. The balance of fluid and osmotic pressures across active biological membranes with application to the corneal endothelium. PloS one, 2015;10(12): e0145422.
Sacco R, Guidoboni G, Jerome JW, Bonifazi G, Marazzi NM, Verticchio Vercellin AC, et al. A theoretical approach for the electrochemical characterization of ciliary epithelium. Life, 2020;10(2): 8.