Keywords
Abstract
Purpose: To present the cyclic strains developed in the lamina cribrosa due to the cardiac cycle-driven fluctuations in the pressure conditions around the optic nerve head.
Design: Finite element analysis on 3-D models of the human eye.
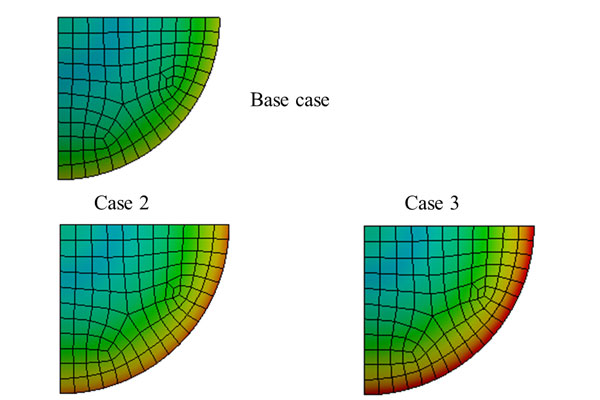
Methods: Varying intraocular pressure and cerebrospinal fluid pressure over a cardiac cycle were provided as boundary conditions in the finite element models. The cyclic strains generated in the lamina cribrosa were compared at different
mean intraocular pressures representing normal and pathological conditions.
Results: The peak maximum principal strains varied from 0.7% to 1.4% across all cases of normal and elevated intraocular pressure, and occurred along the periphery of the lamina cribrosa. The amplitude of the cyclic strains in the lamina cribrosa increased by 3.5% from the normal case to the pathological cases. The amplitudes did not change significantly for the pathological cases with mean intraocular pressures of 21.6 mmHg, 26.6 mmHg, and 31.6 mmHg.
Conclusion and future perspective: The effect of short-term pressure changes on the tissues of the optic nerve head has not been studied extensively. In vitro and ex vivo experiments can be designed based on the results of computational studies to observe the eff ect of cyclic strains on mechanosensitive cells in the optic nerve head. Furthermore, the repetitive impact of cyclic strains in the lamina cribrosa over numerous cardiac cycles gives rise to the possibility of mechanical fatigue contributing to the structural damage around the optic nerve head. A cumulative damage model can be developed based on the results of this study.
References
Weinreb RN, Khaw PT. Primary open-angle glaucoma. The Lancet. 2004;94221711-1720.
Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;4359-393. h
Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol Vis Sci. 1974;10771-783.
Quigley H, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol Vis Sci. 1976;8606-616.
Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma: II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;4635-649.
Wostyn P, De Groot V, Audenaert K, De Deyn PP. Are intracranial pressure fluctuations important in glaucoma? Med Hypotheses. 2011;4598-600.
Kaskar OG, Fleischman D, Lee YZ, Thorp BD, Kuznetsov AV, Grace L. Identifying the Critical Factors Governing Translaminar Pressure Differential Through a Compartmental Model. Invest Ophthalmol Vis Sci. 2019;83204-3214.
Morgan WH, Lind CR, Kain S, Fatehee N, Bala A, Yu D. Retinal vein pulsation is in phase with intracranial pressure and not intraocular pressure. Invest Ophthalmol Vis Sci. 2012;84676-4681.
Downs JC. Optic nerve head biomechanics in aging and disease. Exp Eye Res. 2015;19-29.
Jin Y, Wang X, Zhang L, et al. Modeling the origin of the ocular pulse and its impact on the optic nerve head. Invest Ophthalmol Vis Sci. 2018;103997-4010.
Bellezza AJ, Hart RT, Burgoyne CF. The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci. 2000;102991-3000.
Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Finite element modeling of optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2004;124378-4387.
Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;114189-4199.
Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part I: IOP-induced deformations and influence of geometry. Biomech Model Mechanobiol. 2009;285-98.
Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part II: influence of material properties. Biomech Model Mechanobiol. 2009;299-109.
Norman RE, Flanagan JG, Sigal IA, Rausch SM, Tertinegg I, Ethier CR. Finite element modeling of the human sclera: influence on optic nerve head biomechanics and connections with glaucoma. Exp Eye Res. 2011;14-12.
Leung LK, Ko MW, Lam DC. Effect of age-stiffening tissues and intraocular pressure on optic nerve damages. Mol Cell Biomech. 2012;2157.
Kharmyssov C, Abdildin YG, Kostas KV. Optic nerve head damage relation to intracranial pressure and corneal properties of eye in glaucoma risk assessment. Med Biol Eng Comput. 2019;71591-1603.
Feola AJ, Myers JG, Raykin J, et al. Finite element modeling of factors influencing optic nerve head deformation due to intracranial pressure. Invest Ophthalmol Vis Sci. 2016;41901-1911.
Wang X, Rumpel H, Lim WEH, et al. Finite element analysis predicts large optic nerve head strains during horizontal eye movements. Invest Ophthalmol Vis Sci. 2016;62452-2462.
Hua Y, Tong J, Ghate D, Kedar S, Gu L. Intracranial pressure influences the behavior of the optic nerve head. J Biomech Eng. 2017;3031003.
Shin A, Yoo L, Park J and Demer JL. Finite element biomechanics of optic nerve sheath traction in adduction. J Biomech Eng. 2017;10101010.
Hua Y, Voorhees AP, Sigal IA. Cerebrospinal fluid pressure: revisiting factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2018;1154-165.
Tong J, Ghate D, Kedar S, Gu L. Relative Contributions of Intracranial Pressure and Intraocular Pressure on Lamina Cribrosa Behavior. J Ophthalmol. 2019 Mar 17;2019:3064949.
Muñoz-Sarmiento DM, Rodríguez-Montaño ÓL, Alarcón-Castiblanco JD, Gamboa-Márquez MA, Corredor-Gómez JP, Cortés-Rodríguez CJ. A finite element study of posterior eye biomechanics: The influence of intraocular and cerebrospinal pressure on the optic nerve head, peripapillary region, subarachnoid space and meninges. Informatics in Medicine Unlocked. 2019;100185.
Hasenfratz G. Experimental studies on the display of the optic nerve. In: Ossoinig KC, editor. Ophthalmic echography. The Hague: Martinus Nijhoff/W Junk, 1987:587–602.
Reina MA, Casasola ODL, López A, De Andrés JA, Mora M, Fernández A. The origin of the spinal subdural space: ultrastructure findings. Anesthesia & Analgesia. 2002;4991-995.
Schultz DS, Lotz JC, Lee SM, Trinidad ML, Stewart JM. Structural factors that mediate scleral stiffness. Invest Ophthalmol Vis Sci. 2008;104232-4236.
Spoerl E, Boehm AG, Pillunat LE. The influence of various substances on the biomechanical behavior of lamina cribrosa and peripapillary sclera. Invest Ophthalmol Vis Sci. 2005;41286-1290.
Hou R, Zhang Z, Yang D, et al. Intracranial pressure (ICP) and optic nerve subarachnoid space pressure (ONSP) correlation in the optic nerve chamber: the Beijing Intracranial and Intraocular Pressure (iCOP) study. Brain Res. 2016;201-208.
Morgan WH, Yu D, Cooper RL, Alder VA, Cringle SJ, Constable IJ. The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci. 1995;61163-1172.
Morgan WH, Yu D, Alder VA, et al. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci. 1998;81419-1428.
Liu D, Kahn M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am J Ophthalmol. 1993;5548-556.
Moller PM. The pressure in the orbit. Acta Ophthalmol. 1955;Suppl 431-100.
Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol. 1989;2186-188.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;101268-1279.
Siaudvytyte L, Januleviciene I, Daveckaite A, et al. Literature review and meta-analysis of translaminar pressure difference in open-angle glaucoma. Eye. 2015;101242-1250.
Jonas JB, Berenshtein E and Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci. 2003;125189-5195.
Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;2120-132.
Jay JL, Murdoch JR. The rate of visual field loss in untreated primary open angle glaucoma. Br J Ophthalmol. 1993;3176-178.
Leske MC, Connell AM, Wu S, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. Arch Ophthalmol. 2001;189-95.
Grytz R, Girkin CA, Libertiaux V, Downs JC. Perspectives on biomechanical growth and remodeling mechanisms in glaucoma. Mech Res Commun. 2012;92-106.
Albon J, Purslow PP, Karwatowski WS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;3318-323.
Geraghty B, Jones SW, Rama P, Akhtar R, Elsheikh A. Age-related variations in the biomechanical properties of human sclera. J Mech Behav Biomed Mater. 2012;181-191.
Albon J, Karwatowski WS, Avery N, Easty DL, Duance VC. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995;4368-375.
Simo JC. On a fully three-dimensional finite-strain viscoelastic damage model: formulation and computational aspects. Comput Methods Appl Mech Eng. 1987;2153-173.
Rodríguez JF, Cacho F, Bea JA, Doblaré M. A stochastic-structurally based three dimensional finite-strain damage model for fibrous soft tissue. J Mech Phys Solids. 2006;4864-886.
Martin C, Sun W. Modeling of long-term fatigue damage of soft tissue with stress softening and permanent set effects. Biomech Model Mechanobiol. 2013;4645-655.
Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;107365-7375.
Resta V, Novelli E, Vozzi G, et al. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;92741-2754.
Caprioli J, Coleman AL. Intraocular pressure fluctuation: a risk factor for visual field progression at low intraocular pressures in the Advanced Glaucoma Intervention Study. Ophthalmology. 2008;71123-1129. e3.
Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. 2007;81010-1013.
Kim JH, Caprioli J. Intraocular pressure fluctuation: is it important? J Ophthalmic Vis Res. 2018;2170.
Guo Z, Chang K, Wei X. Intraocular pressure fluctuation and the risk of glaucomatous damage deterioration: a meta-analysis. Int J Ophthalmol. 2019;1123.
Matlach J, Bender S, König J, Binder H, Pfeiffer N, Hoffmann EM. Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression. Clin Ophthalmol. 2019;9.
Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;2134-142.
Jonas JB, Budde WM, Stroux A, Oberacher-Velten IM, Jünemann A. Diurnal intraocular pressure profiles and progression of chronic open-angle glaucoma. Eye. 2007;7948-951.
Lee YR, Kook MS, Joe SG, et al. Circadian (24-hour) pattern of intraocular pressure and visual field damage in eyes with normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2012;2881-887.
Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP. Intracranial pressure fluctuations: a potential risk factor for glaucoma. Acta Ophthalmol. 2015;1e83-e84.
McMonnies CW. The interaction between intracranial pressure, intraocular pressure and lamina cribrosal compression in glaucoma. Clin Exp Optom. 2016;3219-226.
Downs JC, Girkin CA. Lamina cribrosa in glaucoma. Curr Opin Ophthalmol. 2017;2113.
Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O’Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol Vis. 2005;798e810.
Coudrillier B, Boote C, Quigley HA, Nguyen TD. Scleral anisotropy and its effects on the mechanical response of the optic nerve head. Biomechanics and modeling in mechanobiology. 2013;5941-963.
Pijanka JK, Coudrillier B, Ziegler K, et al. Quantitative mapping of collagen fiber orientation in non-glaucoma and glaucoma posterior human sclerae. Invest Ophthalmol Vis Sci. 2012;95258-5270.
Zhang W, Liu Y, Kassab GS. Viscoelasticity reduces the dynamic stresses and strains in the vessel wall: implications for vessel fatigue. Am J Physiol Heart Circ Physiol. 2007;4H2355-H2360.